Follow us for updates:LinkedIn
Aiming to Improve the Lives of Patients Undergoing Vascular Surgery
About VenoStent
A Clinical-Stage Therapeutic Medical Device Company
We’re a clinical-stage therapeutic medical device company that’s developed finely-tuned, bioabsorbable wraps intended to improve outcomes for the 5 million vascular surgeries performed each year.
Technology
A Novel Technology with High Potential
We’re developing a bioabsorbable perivascular wrap, SelfWrap, that goes around arteriovenous (AV) access sites at the time of AV creation surgery. The wrap is intended to improve the usability (i.e. maturation) and durability (i.e. patency) of these sites for chronic kidney disease (CKD) patients requiring hemodialysis. If successful, this could improve the quality of life for CKD patients requiring hemodialysis.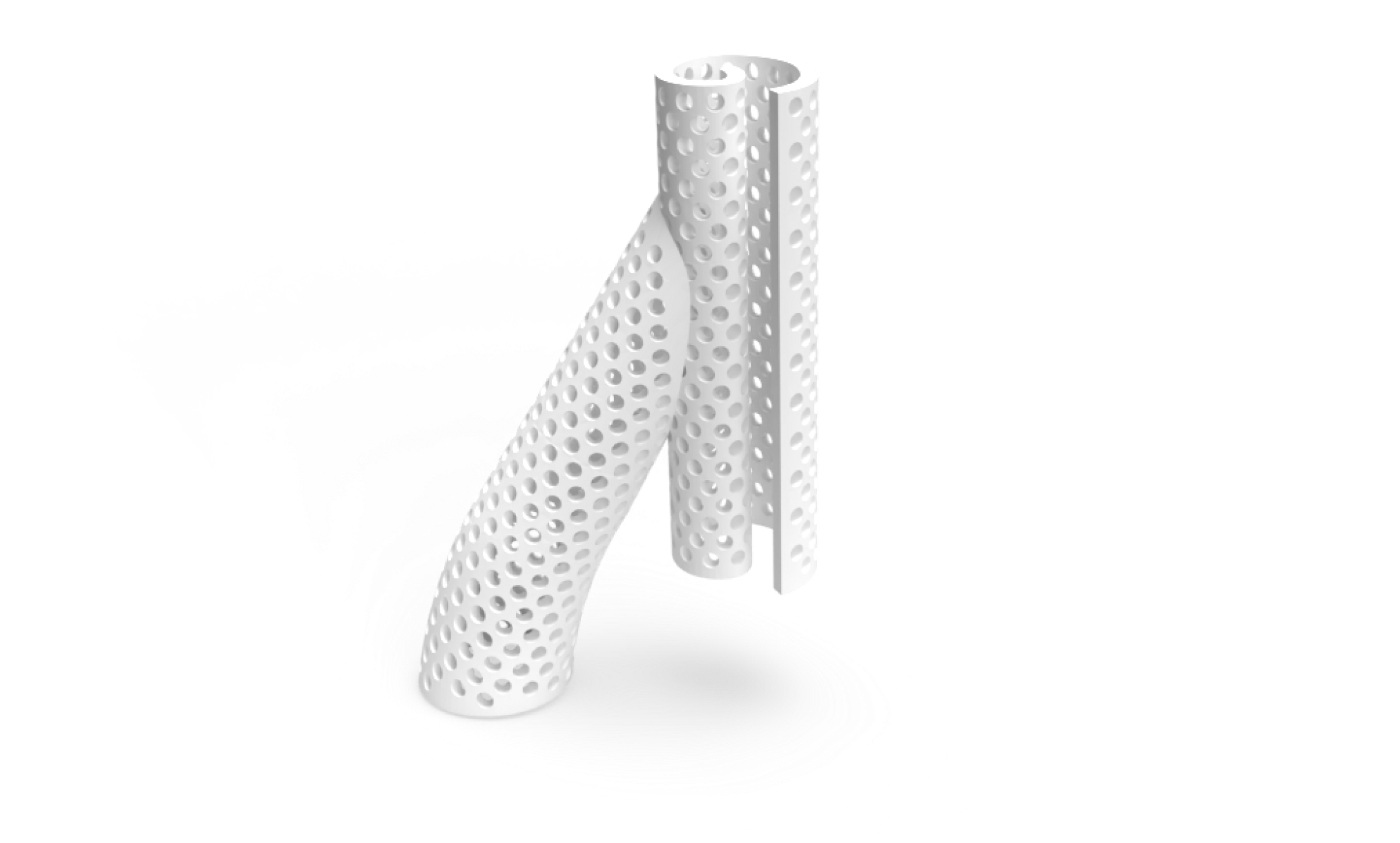

Bioabsorbable
Materials are absorbed by the body, unlike alloy-based products

Finely-Tuned Materials
A conforming fit for each patient

3D-Printed Polymers
Reliably and robustly produced

Numerous Applications
Potential for coronary and peripheral bypass grafting, others
Team
A Cross-Disciplinary Team of Experts

Tim Boire, PhD
CEO & Co-founder

Geoff Lucks
COO & Co-founder

Pamela Misajon
Chief Compliance Officer

Mark Barakat, MD
Director of Clinical Affairs
Tina McArthur
Vice President of Research and Engineering
Partners
















Latest News & Press
VenoStent Enrolls First Subjects in 200-Subject US Clinical Trial
Yahoo Finance |Jan 30, 2024
VenoStent Completes $16M Series A Financing, Receives IDE Approval from FDA to Begin US Trial
Yahoo Finance |Jul 31, 2023
VenoStent Technology Receives Breakthrough Device Designation by FDA
AP |May 17, 2022
SelfWrap is currently in the research and development phase and is not available for sale in any country. Investigational Device. Limited by Federal Law to Investigational Use.
COI Policy© 2025 VenoStent, Inc. All rights reserved.
Contact Usinfo@venostent.com2450 Holcombe Blvd, Suite J
Houston, TX 77021Follow Us